The Strano research group applies chemical engineering analysis and novel tools in nanotechnology to further the understanding and engineering of plant systems. We have developed mathematical theories of how natural and synthetic nanoparticles traffic within living plant tissues. We have also developed the concept of the plant signaling waveform – a temporal concentration wave used by plants to signal internally and externally, conveying enormous information content.
Research Overview
Plants possess remarkable abilities such as self-repair, autonomous growth, and photosynthesis, which allow them to thrive in dynamic environments. Their capacity to monitor and respond to environmental changes through complex signaling mechanisms—such as nutrient and water uptake through roots and gas exchange via stomata—makes them fascinating candidates for an interface with nanotechnology. Plant nanotechnology, particularly the emerging field of plant nanobionics, seeks to leverage these natural capabilities by integrating nanoparticles and sensors to enhance plant functions and gain deeper insights into their physiological processes.
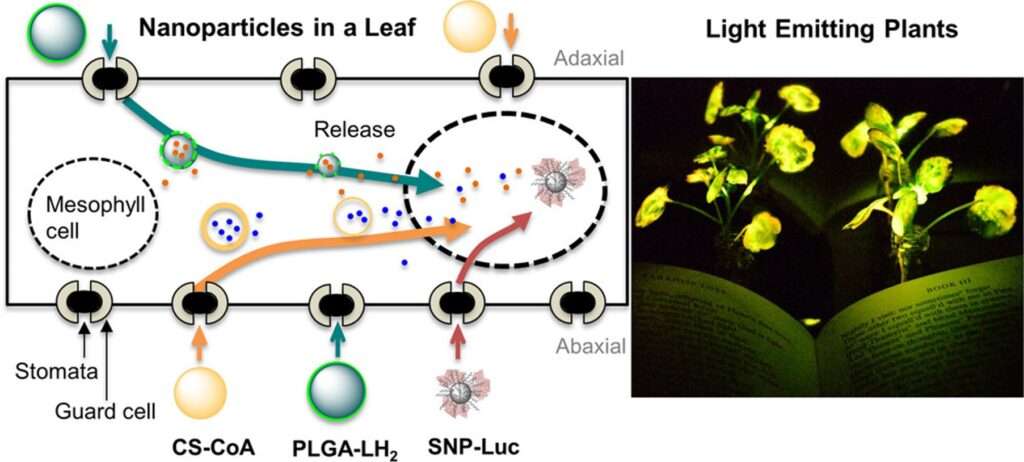 Strano group research spans several exciting areas, including the development of nanoparticles for efficient transport and localization within plant tissues, methods for delivering genetic material to modify plant traits, and innovative projects such as creating light-emitting plants. We can introduce optical nanosensors to phytohormones and environmental analytes to a wide range of plants to monitor crop health and the surrounding environment in real time. We have developed luciferase conjugated and luciferin self-releasing nanoparticles to convert innate plant ATP to chemiluminescence for living plant-based lighting. Plants that sense chemicals in the environment have also been generated with nanobionic organisms that can send information to the internet. Turning such sensors inward to study the plant itself has made new tools to understand agriculture and crop health status, providing important information that can be used to help solve longstanding challenges such as environmental remediation and food and energy security. These efforts are pushing the boundaries of plant nanotechnology, offering new possibilities for agricultural innovation, environmental monitoring, and sustainable biotechnology.
Strano group research spans several exciting areas, including the development of nanoparticles for efficient transport and localization within plant tissues, methods for delivering genetic material to modify plant traits, and innovative projects such as creating light-emitting plants. We can introduce optical nanosensors to phytohormones and environmental analytes to a wide range of plants to monitor crop health and the surrounding environment in real time. We have developed luciferase conjugated and luciferin self-releasing nanoparticles to convert innate plant ATP to chemiluminescence for living plant-based lighting. Plants that sense chemicals in the environment have also been generated with nanobionic organisms that can send information to the internet. Turning such sensors inward to study the plant itself has made new tools to understand agriculture and crop health status, providing important information that can be used to help solve longstanding challenges such as environmental remediation and food and energy security. These efforts are pushing the boundaries of plant nanotechnology, offering new possibilities for agricultural innovation, environmental monitoring, and sustainable biotechnology.
Nanoparticle Transport within Living Plants
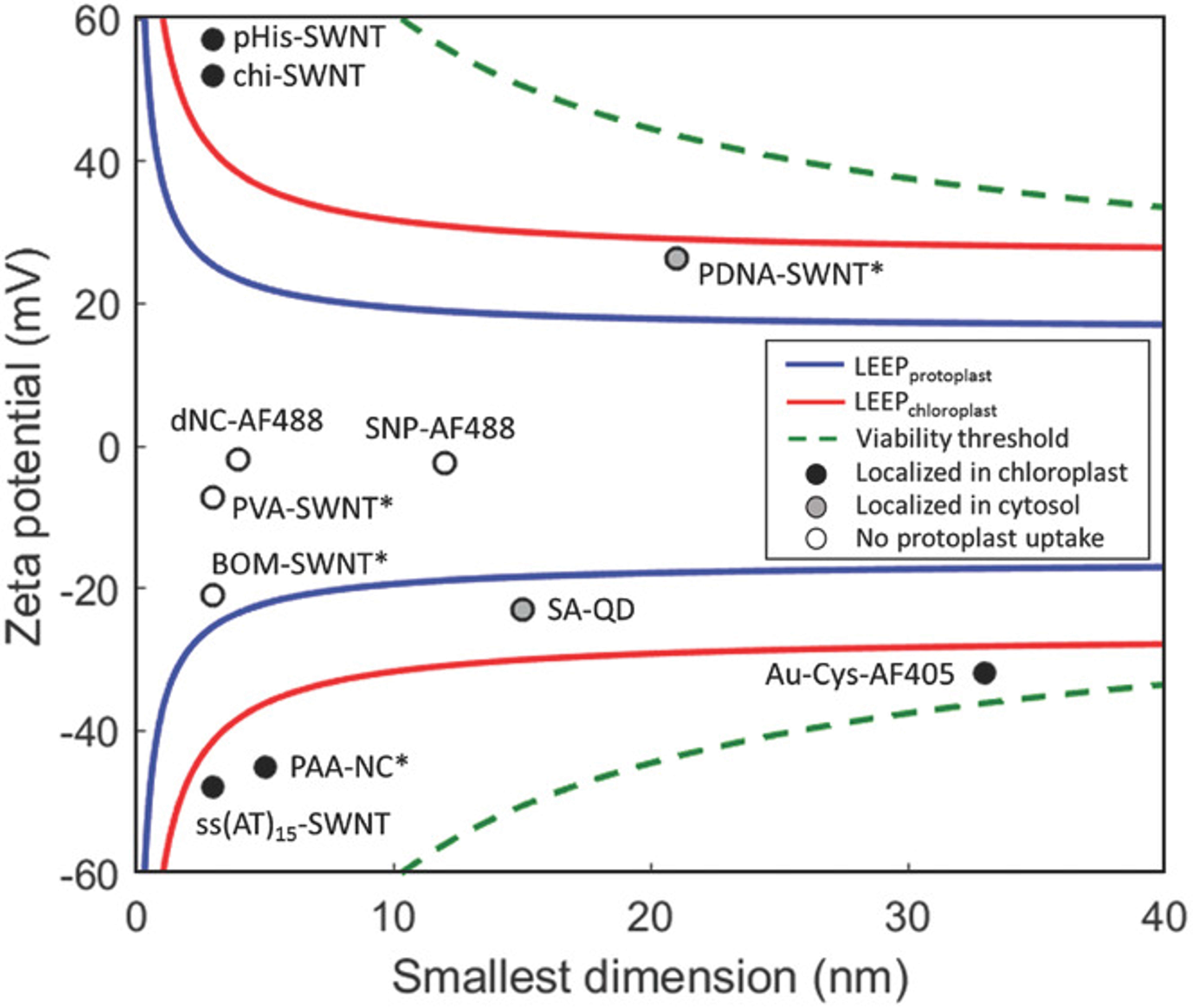
The ability to control the subcellular localization of nanoparticles within living plants offers unique advantages for targeted biomolecule delivery and enables important applications in plant bioengineering. We have developed an experimentally validated mathematical model of lipid exchange envelope penetration mechanism generic for various nanoparticles for plant chloroplasts and protoplasts, which predicts that the subcellular distribution of nanoparticles in plant cells is dictated by the particle size and the magnitude of the zeta potential. Our work can guide the rational design of nanoparticles for targeted delivery into specific compartments within plant cells without the use of chemical or mechanical aid, enabling chloroplast-selective gene delivery nanocarrier and nanosensors.
Reference
Mathematical Modeling of Signaling in Planta
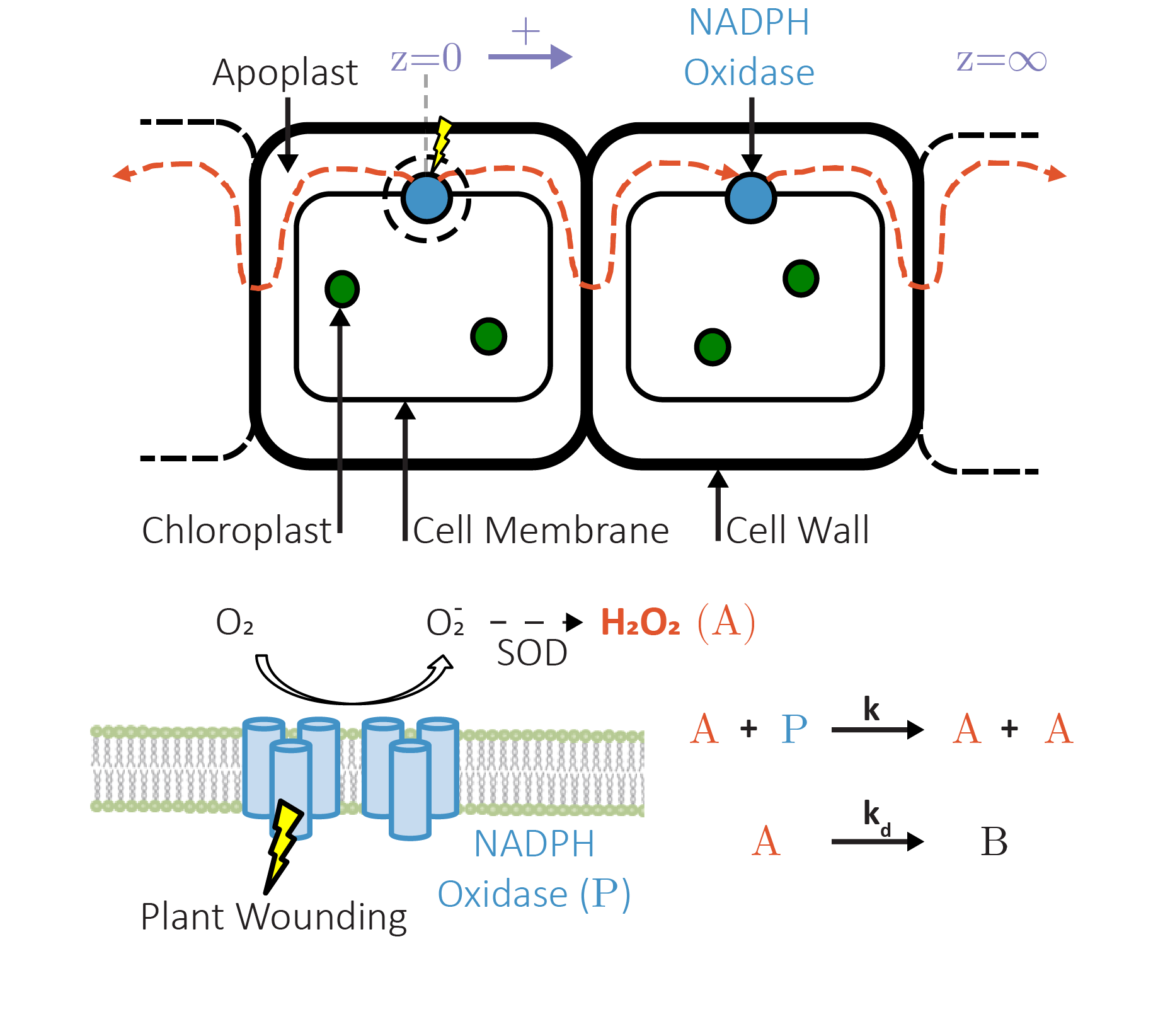
Plants, as sessile organisms, must swiftly adapt to changing environmental conditions through intricate signaling mechanisms. We focus on the development and implementation of single-walled carbon nanotube (SWNT)-based sensors, which enable the real-time, non-destructive monitoring of key signaling molecules like hydrogen peroxide (H₂O₂) and various phytohormones in living plants. These sensors are both reversible and photostable, allowing us to capture dynamic spatiotemporal patterns of chemical signaling initiated by environmental stimuli.
By integrating nanosensor data with mathematical models, we have made significant strides in understanding how plants respond to stress. For example, our mechanical stress model provides a quantitative description of the H2O2 traveling waveform signal generated immediately after wounding. Additionally, multiplexed sensor data have enabled us to characterize the interaction between H₂O₂ and the hormone salicylic acid (SA) under different stress conditions, yielding insights into how plants perceive and respond to different stress conditions such as wounding, bacterial infection, high heat, and high light. We have also developed a transport model for the growth hormone auxin, revealing how its distribution is modulated by key structural features in leaves. Our research highlights the potential of nanosensors to unlock new perspectives on plant signaling and the power of mathematical modeling in formulating hypotheses about the underlying mechanisms driving these processes.
Reference
1) Ang, M. C. Y., Saju, J. M., Porter, T. K., Mohaideen, S., Sarangapani, S., Khong, D. T., … & Sarojam, R. (2024). Decoding early stress signaling waves in living plants using nanosensor multiplexing. Nature Communications, 15(1), 2943.
3) Lew, T. T. S., Koman, V. B., Silmore, K. S., Seo, J. S., Gordiichuk, P., Kwak, S. Y., … & Strano, M. S. (2020). Real-time detection of wound-induced H2O2 signalling waves in plants with optical nanosensors. Nature plants, 6(4), 404-415. 4) Khong, D. T., Vu, K. V., Sng, B. J. R., Choi, I. K. Y., Porter, T. K., Cui, J., … & Jang, I. C. (2024). A Near Infrared Fluorescent Nanosensor for Spatial and Dynamic Measurements of Auxin, Indole-3-Acetic Acid, in Planta. bioRxiv, 2024-05.
Nanosensors in Planta - Early Stress Detection
Nanosensors embedded in plants offer a revolutionary approach to real-time monitoring of biochemical signals, allowing us to detect early stress responses before visible symptoms appear. These sensors are designed to detect critical signaling molecules such as hydrogen peroxide (H₂O₂), salicylic acid (SA), and gibberellins (GAs), all of which play key roles in how plants react to environmental stressors. By utilizing nanomaterials like carbon nanotubes, the sensors are non-invasive and capable of reporting dynamic molecular changes within plants under various stress conditions such as heat, light, and bacterial infection.
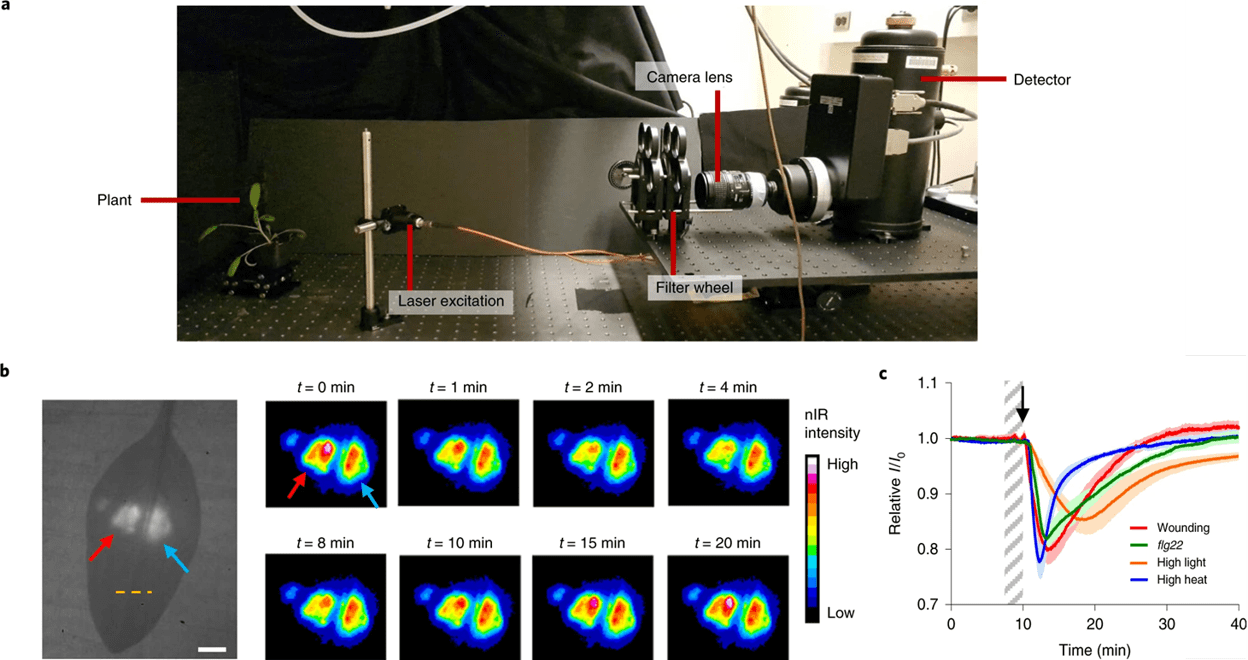
Figure 1. a, Photograph of the experimental setup for the in vivo standoff detection. b, Left: brightfield image of spinach leaf in the intact plant infiltrated with the active and reference sensor under laser excitation. Red and blue arrows indicate H2O2 sensor and reference, respectively; the orange dashed line represents wounding inflicted on the leaf. Scale bar, 5 mm. Right: false-coloured images show the quenching response of H2O2 sensor, while reference intensity remained invariant after wounding. Time (t) denotes the time points after wounding. c, Nanosensor response after the application of other stresses such as flg22, high light and heat stress treatments.
The nanosensors function by specifically binding to their target molecules, triggering a fluorescent signal that can be detected externally through infrared imaging. In one study, our H₂O₂ nanosensor revealed distinct stress responses across multiple plant species, offering valuable insights into how plants adapt to various environmental challenges. Building on this work, we later multiplexed the H₂O₂ sensor with a salicylic acid (SA) sensor, allowing us to track the timing and patterns of these signaling molecules in response to diverse stressors. This dual approach unveiled stress-specific “fingerprints” unique to each type of threat.
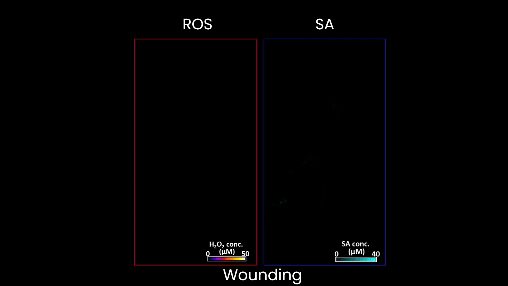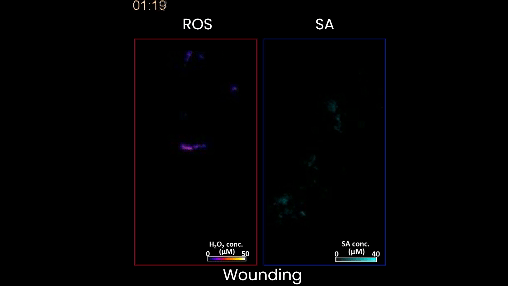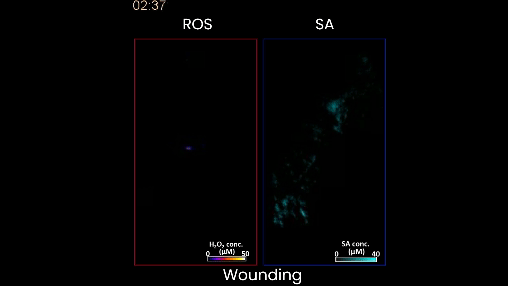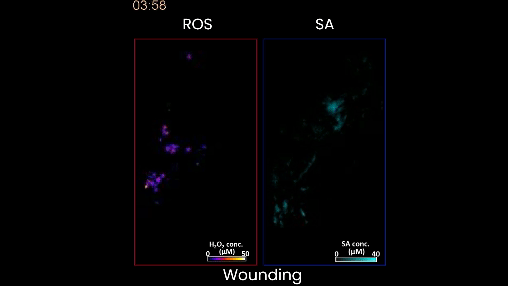
Figure 2. When the plant is wounded, as in this case, the left shows a moving waveform in response, but there is very little production of salicylic acid. This is the stress signature of wounding. In contrast, when the plant is stressed by too much heat or light, or a bacterial infection, different waveforms of salicylic acid accompany of the hydrogen peroxide wave on the left.
These findings demonstrate that plants release these molecules at different intervals depending on the type of stress, forming distinct patterns that serve as early warning systems. These molecular signatures enable farmers to detect potential threats to their crops, allowing for timely interventions before significant damage occurs. Beyond providing critical insights into plant stress management, these nanosensors have the potential to revolutionize agriculture by supporting the development of climate-resilient crops and enhancing advanced farming practices.
Check out our work in MIT News!
Reference
1) Giraldo, J. P., Landry, M. P., Faltermeier, S. M., McNicholas, T. P., Iverson, N. M., Boghossian, A. A., … & Strano, M. S. (2014). Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nature materials, 13(4), 400-408.
3) Ang, M. C. Y., Saju, J. M., Porter, T. K., Mohaideen, S., Sarangapani, S., Khong, D. T., … & Sarojam, R. (2024). Decoding early stress signaling waves in living plants using nanosensor multiplexing. Nature Communications, 15(1), 2943. 4) Lew, T. T. S., Koman, V. B., Silmore, K. S., Seo, J. S., Gordiichuk, P., Kwak, S. Y., … & Strano, M. S. (2020). Real-time detection of wound-induced H2O2 signalling waves in plants with optical nanosensors. Nature plants, 6(4), 404-415.
Disruptive & Sustainable Technologies for Agricultural Precision (DiSTAP)
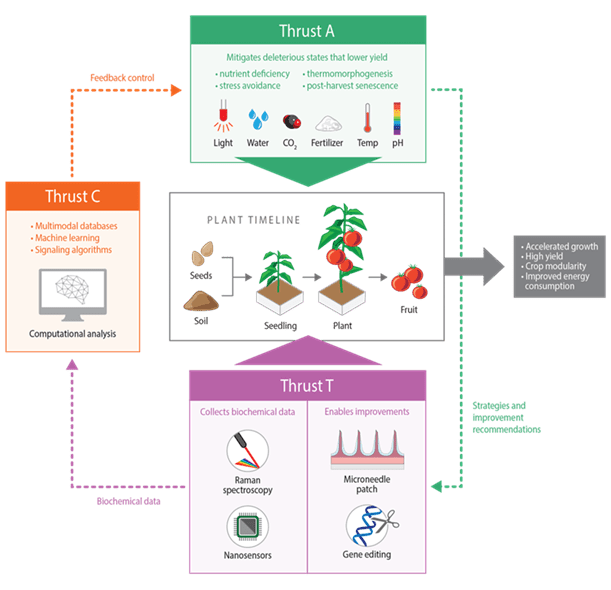
DiSTAP is a cutting-edge research program launched in 2018 as part of the Singapore-MIT Alliance for Research and Technology (SMART) Centre. Our multidisciplinary team focuses on enhancing agricultural precision by creating advanced tools and methods for real-time monitoring, data-driven decision-making, and improved crop management. DiSTAP integrates expertise from plant biology, engineering, and data science to address critical challenges in global food security and environmental sustainability. By leveraging groundbreaking innovations such as novel sensors, nanotechnology, and AI-driven analytics, we aim to increase crop yields, reduce resource consumption, and promote sustainable farming practices worldwide. DiSTAP is at the forefront of addressing critical challenges in global food security and environmental sustainability. [Link]