The Strano research group leverages advances in synthetic techniques and mechanistic modeling to create materials with unique and unprecedented combinations of properties. Areas include two-dimensionally structured polyaramids, and new characterization and processing tools for them.
Research Overview
 Nanotechnology enables new types of catalytic templating not otherwise accessible, particularly for new materials discovery. In this area, the Strano research group has been interested in generating novel 2D materials from organic, solution phase processing, as opposed to the limitations of Chemical Vapor Deposition. Work includes new theories of solution phase polymerization of 2D monomers, and the experimental realization of 2D polyaramids. As materials, our work has shown that 2D polyaramids exist between polymeric materials and 2D inorganic crystalline materials, combining the properties of both, but with a solution-phase synthetic route. 2DPA-1 is a melamine and acyl chloride polycondensation that produces a material that is stronger than steel, is as light as a conventional plastic, and has properties that mimic materials like graphene.
Nanotechnology enables new types of catalytic templating not otherwise accessible, particularly for new materials discovery. In this area, the Strano research group has been interested in generating novel 2D materials from organic, solution phase processing, as opposed to the limitations of Chemical Vapor Deposition. Work includes new theories of solution phase polymerization of 2D monomers, and the experimental realization of 2D polyaramids. As materials, our work has shown that 2D polyaramids exist between polymeric materials and 2D inorganic crystalline materials, combining the properties of both, but with a solution-phase synthetic route. 2DPA-1 is a melamine and acyl chloride polycondensation that produces a material that is stronger than steel, is as light as a conventional plastic, and has properties that mimic materials like graphene.
Our work sits at the intersection of 2D nanotechnology, which includes the study of graphene, hBN, and MoS2, and polymer science, and promises to combine the exciting electrical, mechanical, and gas barrier properties of 2D nanomaterials with the synthetic processability and manufacturability of traditional polymers.
Synthetic Methods for 2D Polyaramids
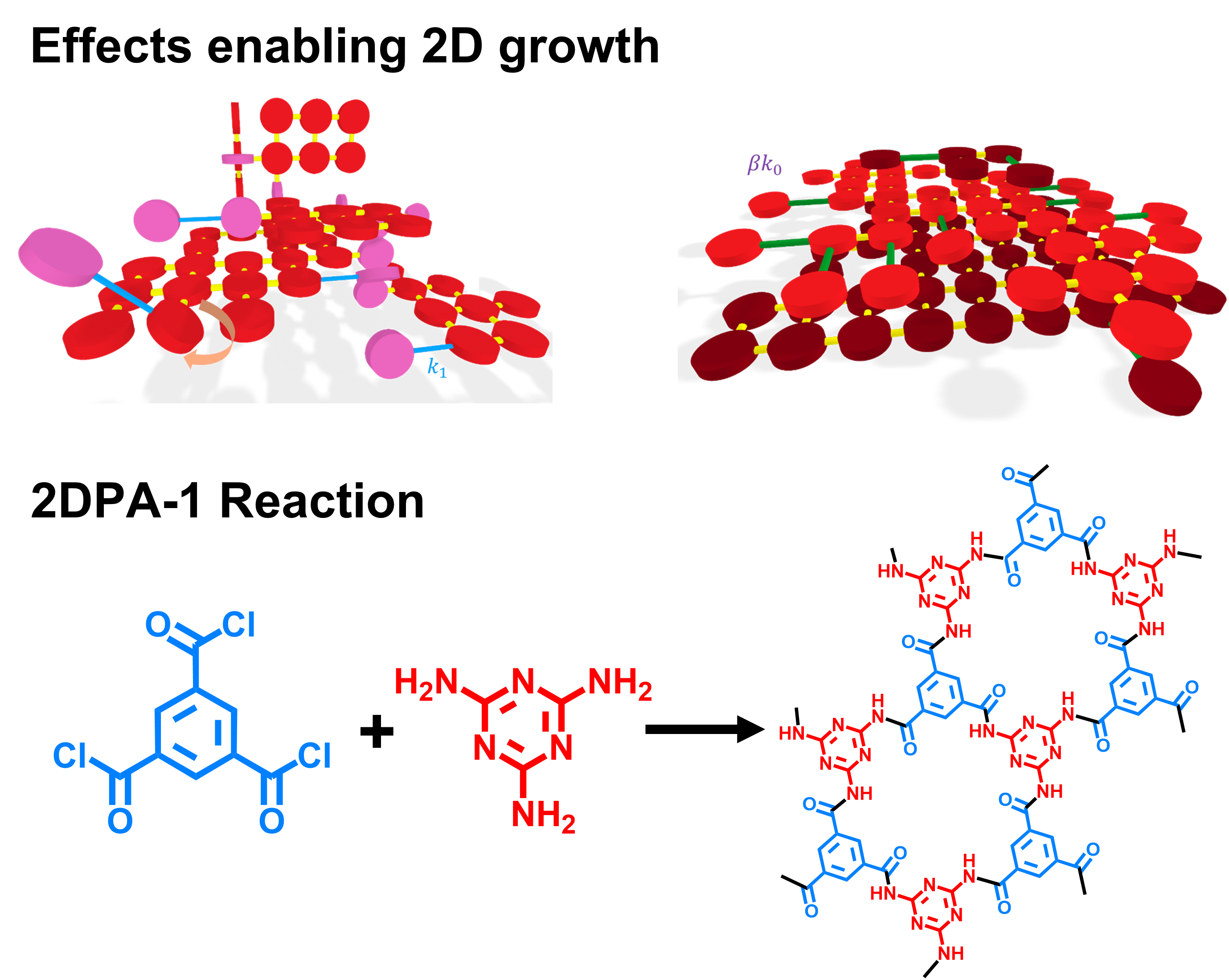
Synthesizing two-dimensional polymers in a homogenous solution is complicated by two major factors. The first is that 2D sheets irreversibly grow into amorphous 3D structures; a single, out-of-plane growth can ruin the 2D structure. The second complication is that the number of reaction sites that result in a 3D polymer will always exceed that for a 2D one. From a geometric perspective, a 2D circular sheet reacts along its perimeter while a 3D sphere reacts along its surface area, and so the 3D sphere will always have more reactive sites than the 2D circular disk.
For 2D polyaramid synthesis, we chose monomers that can have strong intermolecular interactions and rigid bonds. The rigid bonds prevent the polymers from rotating out of plane, into three dimensions, as it grows, while the strong intermolecular interactions enable already-formed 2D sheets to act as a template for more to grow.
2DPA-1 is synthesized by combining the monomers trimesoyl chloride and melamine in NMP and pyridine at atmospheric temperature and pressure. As the reaction proceeds, a gel forms over time, which is indicative of the strong hydrogen-bonding interactions between our 2D sheets. We then purify the gel and isolate the 2DPA-1 powder, which can be further processed into spin-coated thin films.
Reference
1H-NMR and Diffusion NMR Methods for 2D Polyaramids
2DPAs represent a class of 2D polymers synthesized via irreversible solution-phase polymerization, which do not require a lengthy crystallization process. However, such polymers present a challenge to characterize because of their limited solubilities, rendering them incompatible with chromatography or corrosion-sensitive instruments.
Generally, for polymers, end-group analysis using Nuclear Magnetic Resonance (NMR) spectroscopy stands as a universal method for determining molar masses. NMR spectroscopy here serves as an appealing approach to examine 2DPA end groups owing to its easy accessibility, relatively short measurement times, and quantitative information derived from the signals of unique chemical moieties.
Our 2DPAs are distinctive because they are soluble in trifluoracetic acid (TFA) and dimethyl sulfoxide (DMSO), which are viable options for NMR analysis. We have used 1H NMR analysis of the aromatic and proton end group peak regions to characterize 2DPA growth from monomeric precursors, dendrimers, and other impurities. We have also used this technique to determine the diameter of 2DPA-1 disk-shaped particles, or platelets, with respect to different reaction times.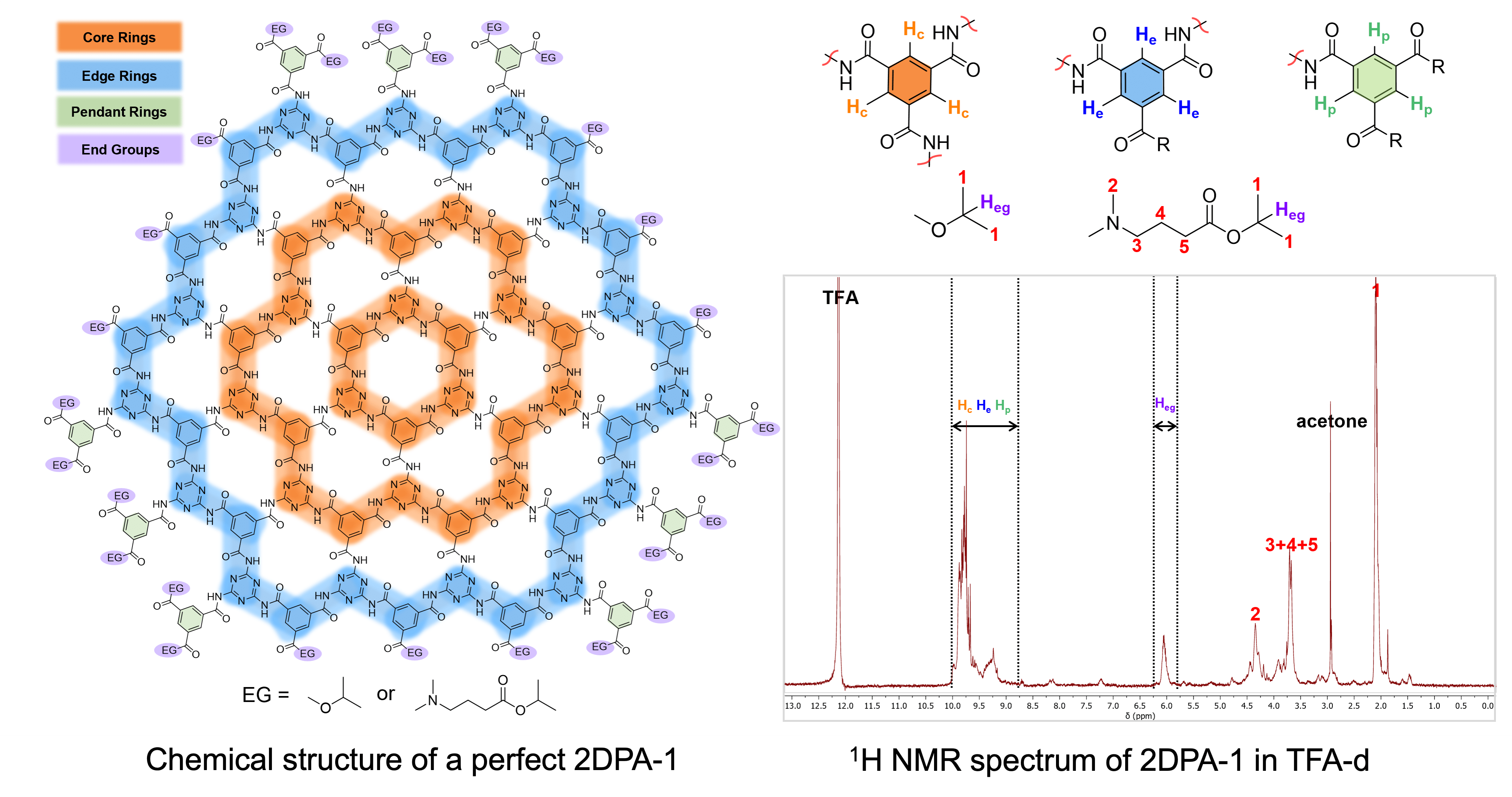
We developed two metrics, r and s, to characterize the growth of our 2DPA samples. The ratio of aromatic-to-end group protons (r) serves as a valuable metric for swiftly determining the diameter of 2DPA platelets and distinguishing between dendrimers and polycyclic aramids. Additionally, the skewness of the aromatic proton peaks (s), allows us to clearly distinguish between dendritic 3D molecules, small polycyclic oligomers, and large polycyclic domains. The combined use of these two metrics enables us to comprehend diverse synthetic approaches and processing conditions. We’ve also derived from planar graph theory how a dendritic 3D polymer and a perfect polycyclic 2D polymer grow with r and s to further discern the properties of our samples.